Celery hydrosol (Apium graveolens) – PrettyWoman.Vn đã tổng hợp thông tin từ nhiều nguồn, giúp bạn có góc nhìn đa chiều hơn. Nào chúng ta bắt đầu thôi
Bài viết Truy cập Mở
Chưng cất tinh dầu từ hạt cần tây ( Apium Graveolens L.) với sự hỗ trợ của sóng siêu âm và đặc tính sinh học và hương thơm của nó
qua

1,*,

2

3

1,*
1
Viện Sản phẩm Tự nhiên và Mỹ phẩm, Khoa Công nghệ Sinh học và Khoa học Thực phẩm, Đại học Công nghệ Lodz, Stefanowskiego 4/10, 90-924 Lodz, Ba Lan
2
Viện Công nghệ Lên men và Vi sinh, Khoa Công nghệ Sinh học và Khoa học Thực phẩm, Đại học Công nghệ Lodz, Wólczańska 171/173, 90-924 Lodz, Ba Lan
3
Viện Phân tích và Công nghệ Thực phẩm, Khoa Công nghệ Sinh học và Khoa học Thực phẩm, Đại học Công nghệ Lodz, Stefanowskiego 4/10, 90-924 Lodz, Ba Lan
*
Các tác giả mà thư từ nên được giải quyết.
Phân tử 2020 , 25 (22), 5322; https://doi.org/10.3390/molecules25225322
Nhận: ngày 14 tháng 10 năm 2020/Sửa đổi: ngày 12 tháng 11 năm 2020/Chấp nhận: ngày 13 tháng 11 năm 2020/Đã xuất bản: 14 tháng 11 năm 2020
(Bài này thuộc Chuyên san Thành phần Hóa học và Hoạt tính Sinh học của Tinh dầu )
Tải xuống
Tải xuống PDF Tải xuống PDF có bìa Tải xuống XML Tải xuống Epub
Duyệt số liệu
Phiên bản Ghi chú
trừu tượng
Mục đích của nghiên cứu là nâng cao hiệu quả của quá trình chưng cất bằng nước và xác định thành phần dễ bay hơi, hoạt tính sinh học và đặc tính mùi thơm của tinh dầu từ hạt cần tây ( Apium Graveolens L.). Tinh dầu được chiết xuất từ nguyên liệu thực vật bằng phương pháp chưng cất siêu âm với hiệu suất cao hơn khi so sánh với phương pháp chưng cất bằng nước cổ điển. Hoạt tính kháng khuẩn được đánh giá bằng phương pháp trở kháng đối với vi khuẩn Pseudomonas aeruginosa, Escherichia coli, Bacillus subtilis, Staphylococcus aureus và nấm men Candida vini cũng như nấm mốc Aspergillus niger và Penicillium expansum .với các giá trị nồng độ ức chế tối thiểu (MIC) (μL/mL): 30, 10, 20, 3, 30, 40 và 40 tương ứng. Dầu sở hữu hoạt tính chống oxy hóa 2,2-diphenyl-1-picrylhydrazyl (DPPH) rất yếu với giá trị nồng độ ức chế tối đa một nửa (IC 50 ) là 81,6 g/L. Các nghiên cứu ban đầu về hồ sơ mùi thơm chỉ ra rằng cảm nhận về mùi thơm của dầu có thể liên quan đến giới tính của những người tham gia hội thảo. Theo phụ nữ, mùi thơm của dầu hạt cần tây giống như mùi thảo mộc. Theo quan điểm của đàn ông, nó có mùi hương tươi mát, rêu và nấm.
từ khóa:
tối ưu hóa ; siêu âm ; hoạt tính sinh học ; phương pháp Taguchi ; hồ sơ hương thơm
1. Giới thiệu
Xem xét tiềm năng sinh học cao của các loại tinh dầu, nên tìm kiếm các phương pháp hiệu quả mới để chiết xuất chúng. Trong nghiên cứu này, nguyên liệu thực vật được xử lý bằng sóng siêu âm nhằm tăng hiệu suất của quá trình chưng cất thủy phân, đồng nghĩa với việc tăng lượng tinh dầu thu được trên một đơn vị trọng lượng của nguyên liệu.
Cơ chế chiết xuất tinh dầu bằng quá trình siêu âm dựa trên hai hiện tượng vật lý chính như sự khuếch tán qua thành và màng tế bào và sự phá hủy cơ học thành tế bào thực vật bằng sóng áp suất và tạo bọt, và tiếp theo là rửa sạch nội dung của tế bào. [ 1 ]. Từ quan điểm kinh tế, việc áp dụng phương pháp chưng cất tinh dầu bằng phương pháp siêu âm có lợi không chỉ ở chỗ hiệu quả chiết xuất cao hơn mà còn tiết kiệm năng lượng, dung môi và thời gian tiêu thụ [ 2]. Do rút ngắn thời gian tiếp xúc của nguyên liệu thô với nhiệt độ cao, quá trình này tránh được những nhược điểm của quá trình chiết xuất tinh dầu thông thường, chẳng hạn như sự hình thành sản phẩm phụ bằng cách phân hủy các hợp chất nhạy cảm với nhiệt và nhiệt [ 3 ] .
Mục tiêu của nghiên cứu này là cải thiện năng suất khai thác tinh dầu bằng cách chưng cất được hỗ trợ bởi sonication của nguyên liệu thô. Không có thông tin trong tài liệu về ảnh hưởng của quá trình sonication của hạt cần tây ( Apium Graveolens L., Apiaceae Lindl.) đối với quá trình chưng cất tinh dầu cũng như chất lượng sản phẩm thu được. Nghiên cứu duy nhất về chiết xuất có hỗ trợ siêu âm được thực hiện bởi Zor et al. (2017), đã được chứng minh là một phương pháp nhanh chóng và hiệu quả để chiết xuất cồn từ hạt cần tây, một nguồn giàu chất chống oxy hóa [ 4 ]. Cần tây và tinh dầu của nó được biết đến với các thuộc tính trị liệu, y tế và công nghiệp [ 5]. Do các hoạt động chống oxy hóa và tác dụng kháng khuẩn chống lại vi khuẩn, nấm men và nấm mốc, dầu hạt cần tây có thể được sử dụng làm chất bảo quản thực phẩm tự nhiên thay thế, thực phẩm chức năng và thành phần dinh dưỡng [ 6 ] . Nghiên cứu được trình bày đặc biệt nhấn mạnh đến hành động môi trường, vì vậy những hạt giống cần tây thải loại không đáp ứng các tiêu chí chất lượng và không có bất kỳ giá trị gieo trồng nào đã được sử dụng.
Để tối đa hóa hiệu quả của quy trình, thí nghiệm đã được lên kế hoạch theo phương pháp thiết kế thử nghiệm (DOE) với việc sử dụng phương pháp Taguchi để tối ưu hóa các thông số của quy trình sonication hạt để tiết kiệm thời gian và tiêu thụ năng lượng. Thành phần định tính và định lượng, các thông số hóa lý, hoạt tính sinh học và cấu hình mùi thơm của tinh dầu thu được từ nguyên liệu thô được siêu âm ở các thông số tối ưu đã được phân tích.
Quá trình chưng cất hydro, trong đó áp dụng sonication của nguyên liệu thô, được đặc trưng bởi sự gia tăng cao về lượng tinh dầu trên một đơn vị trọng lượng của nguyên liệu thô so với quá trình chưng cất hydro. Sản lượng tinh dầu hạt cần tây đã tăng 48,3%.
2. Kết quả và thảo luận
2.1. Phương pháp thiết kế thử nghiệm Taguchi
Kết quả của quá trình tối ưu hóa Taguchi được minh họa trong Bảng 1 . Ở các mức độ làm việc áp dụng riêng, sản lượng tinh dầu thu được từ 1,456 ± 0,011 đến 1,978 ± 0,048 g trên 100 g hạt. Nó đã chỉ ra rằng, giá trị Eta càng cao thì hiệu quả càng cao. Điều này phù hợp với các giả định về hàm mất mát của Taguchi càng lớn càng tốt. Theo kết quả phân tích phương sai (ANOVA), tất cả các thông số được tối ưu hóa đều có ảnh hưởng có ý nghĩa thống kê đến sản lượng tinh dầu thu được ( p <0,05).
Bảng 1. Phân tích thống kê các yếu tố đầu vào cho quá trình tiền xử lý hạt cần tây bằng sóng siêu âm theo phương pháp Taguchi.
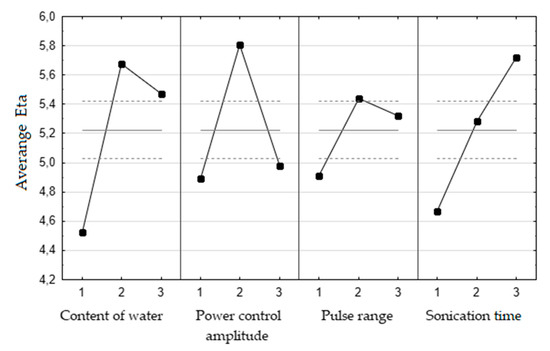
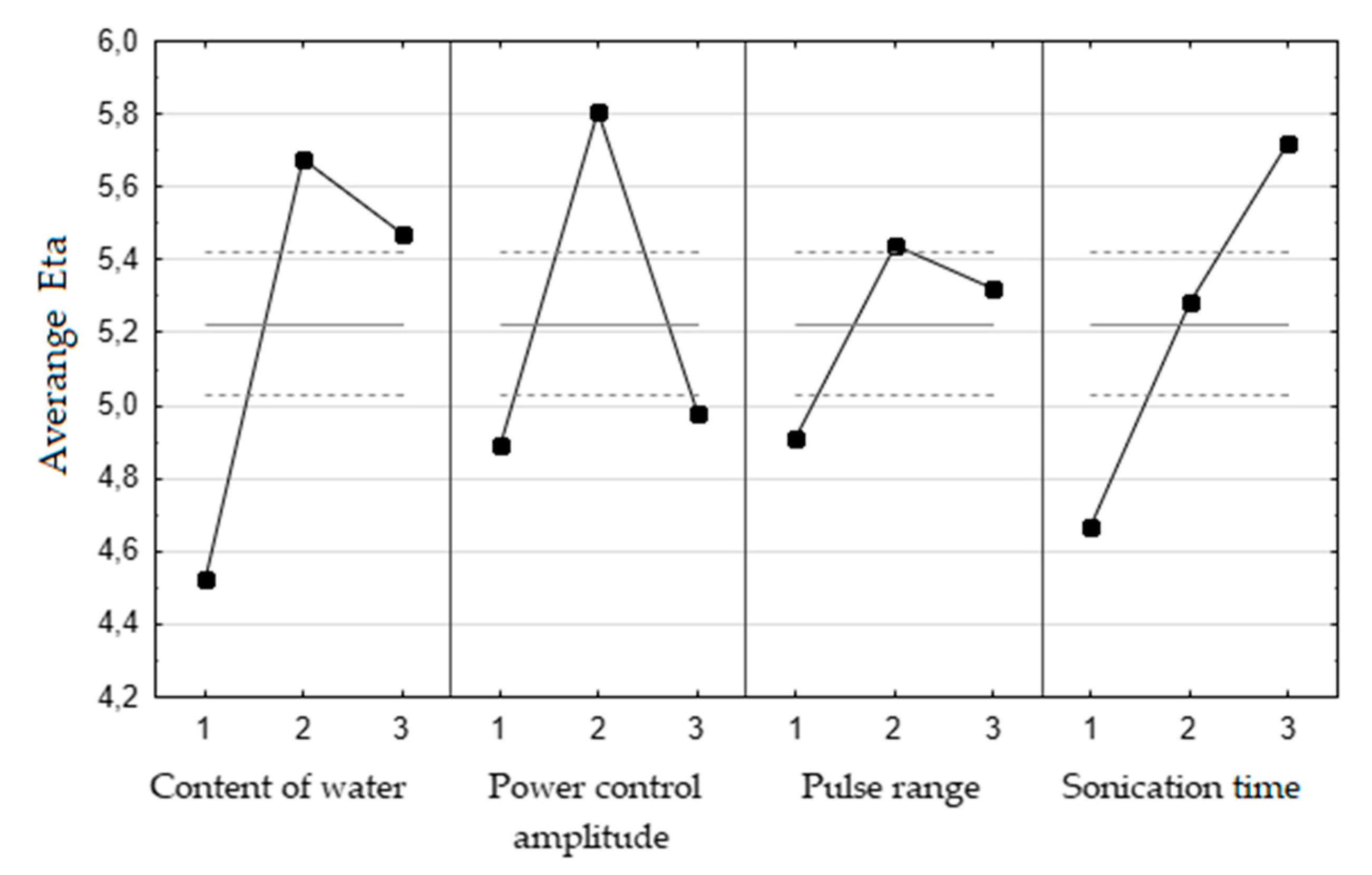
Ban đầu, điểm uốn trên đường cong không thu được trong biểu đồ Eta so với thời gian siêu âm ( Hình 1 ). Do đó, các thí nghiệm bổ sung cho thời gian siêu âm (50, 70 và 90 phút) trong điều kiện tối ưu của các thông số khác đã được thực hiện. Hiệu suất chưng cất thủy phân lần lượt là 2,15 ± 0,032, 2,07 ± 0,06 và 2,04 ± 0,06 g/100 g hạt. Người ta cho rằng thời gian tối ưu của quá trình sonication là 50 phút, do thiếu sự cải thiện hiệu suất đáng kể cũng như các cân nhắc về kinh tế và sinh thái. Các điều kiện tối ưu cho hạt cần tây được xử lý trước bằng siêu âm như sau: thời gian siêu âm 50 phút, dải xung 0,5, biên độ điều khiển công suất 60% và hàm lượng nước 700 mL ( Bảng 2 ) .
Figure 1. The graphs of average Eta versus input levels of optimized parameters. Solid line—value of Eta, dashed line—±2∙standard error, 1,2,3—input levels for each parameters (content of water: 1–350 mL, 2–700 mL, 3–950 mL; power control amplitude 1–20%, 2–60%, 3–100%); pulse range: 1–0.1, 2–0.5, 3–1; sonication time: 1–5 min, 2–20 min, 3–50 min); n = 3.
Table 2. Expected S/N ratio (Eta) under optimum conditions.
2.2. Efficiency of Ultrasonic Hydrodistillation
Statistically calculated expected S/N ratio (Eta) under optimum conditions was 6.971801 (Table 3) which means that, theoretically, under optimal conditions of ultrasound seed treatment, 2.23 g essential oil should be obtained. The experimental yield of essential oil from celery seeds sonicated under optimum conditions equalled 2.15 ± 0.032 g/100 g of seeds. The efficiency increased by 48.3%. The increased efficiency of ultrasound-assisted hydrodistillation results from the specifics of the action of ultrasound [7,8]. The pressure waves and the cavitation led to the mechanical destruction of plant cells, especially cell walls and membranes. The results of this action are the better circulation of extraction solvent, more effective eluting of cell content, and thus more efficient usage of the biological potential of raw material. Another advantage is that sonication accelerates the water absorption leading to faster swelling of the plant material and increasing pore size in the cell walls, and thus facilitates the mass transfer.
Table 3. Composition of essential oil from ultrasounds pre-treated celery seeds (GC-MS).
According to the literature, celery seed contains approximately 2% of volatile oil [9]. Previous literature studies do not show this method of treatment in the case of hydrodistillation of essential oil from celery seeds. However, ultrasound-assisted extraction is a valuable green and novel technique applied in essential oil hydrodistillation [7]. It has been found that ultrasonic hydrodistillation or maceration significantly affected the increase of the efficiency of essential oil from thyme leaves (Thymus vulgaris L.) (about 9%), carrot seeds (Daucus carota L.) (about 33%), peppermint leaves (Mentha piperita L.) (about 10%), and marjoram herb (Origanum majorana L.) (about 12%) [2,10,11].
There was almost a 50% increase in the essential oil efficiency, which confirms the effectiveness of ultrasonic hydrodistillation in the case of extraction of bioactive compounds from hard plant materials like seeds [12]. The process is ecologically significant, because it allows for shortening the distillation stage, electricity, and time consumption.
2.3. Physicochemical Parameters
The physicochemical specification of the essential oil is as follows: density 0.869 ± 0.003 g/mL (20 °C), refractive index n20D n D 20 1.47213 ± 0.0004, and optical density [α]20D [ α ] D 20 + 71.08 ± 0.003.
2.4. Chemical Composition
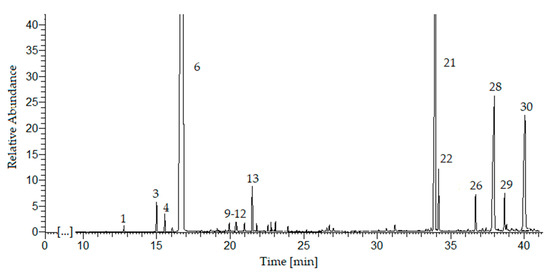
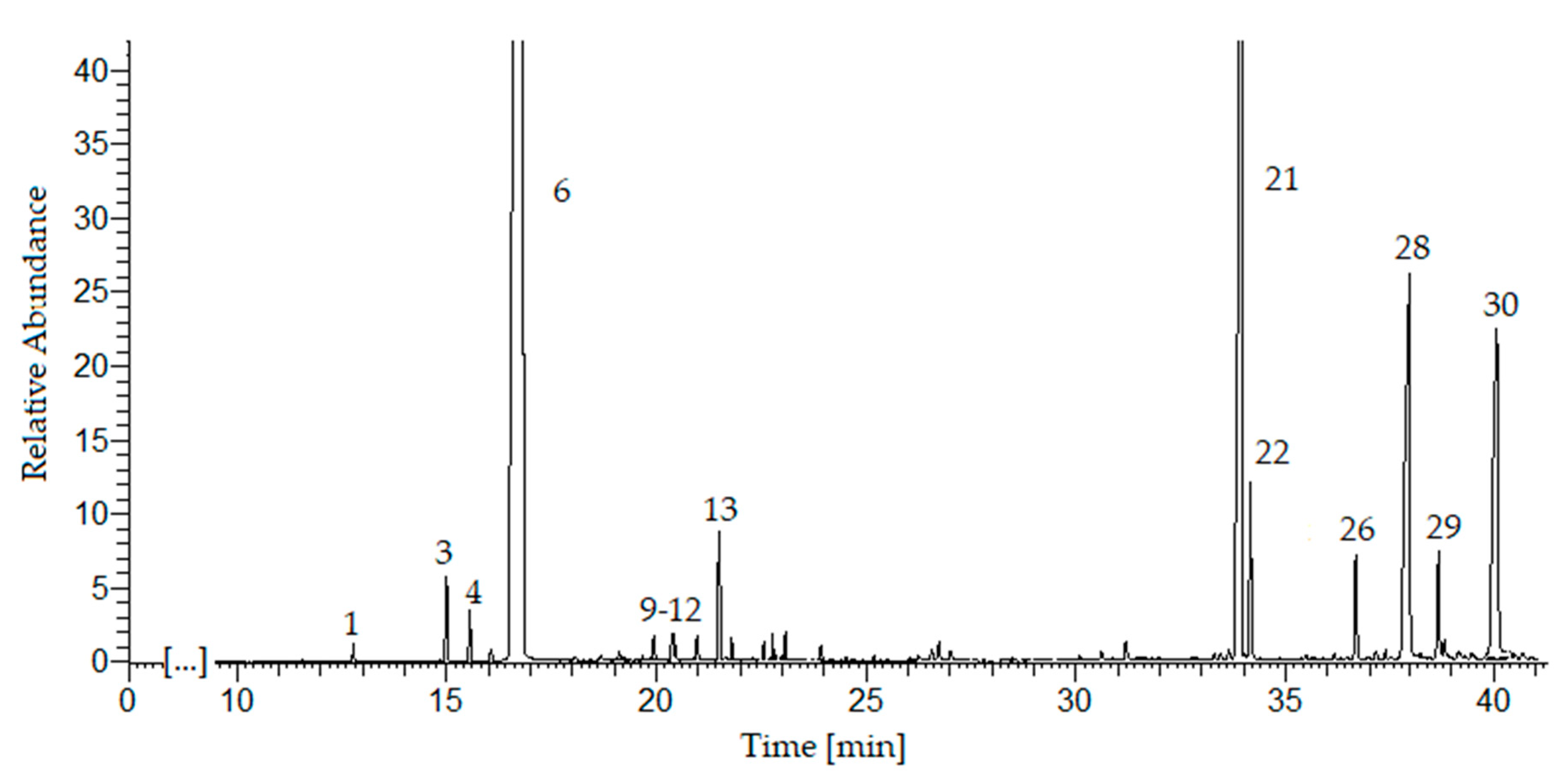
In the essential oil obtained by ultrasonic hydrodistillation, 30 compounds were identified (Table 3, Figure 2) which constitute 99% of the composition (GC-MS, The NIST Library). The principal chemical constituents were limonene (76.9%), β-selinene (9.7%), sedanenolid (3.4%), 3-butylphtalide (3.6%), and α-selinene (1.4%), which is consistent with the literature data [14,15,16]. The monoterpenes (78.4%) were the predominant group of chemical compounds, followed by sesquiterpenes (11.1%) and hydrocarbons (7.8%). The monoterpenoids and sesquiterpenoids have been identified in a small amount (total 1.7%).
Figure 2. GC-MS chromatogram of the essential oil from sonicated celery seeds (Apium graveolens L.). For peak identification, see Table 3.
2.5. Similarity Analysis
Statistical analysis consisting of comparing (correlating) individual spectra and determining the correlation coefficient between near-infrared (NIR) spectra of essential oil from celery seeds with and without ultrasounds pre-treatment was performed. The correlation coefficient was 97.8%, which indicates high similarity. The high similarity was also confirmed in the mid-infrared (MIR) spectroscopy analysis, where the specific locations of the bands were identical for both essential oils. According to the Mann–Whitney test with a significant level of 0.05, the amounts of sedanenolide in essential oil obtained by the hydrodistillation as well as from sonicated celery seeds were statistically significantly different [13]. That may be because the sedanenolide, just as some of the volatile phthalides, is unstable due to its active dihydrobenzene structure [17]. Accordingly, decomposition of this compound could have occurred at high temperatures accompanying the hydrodistillation or sonication processes.
2.6. Antimicrobial Activity
The essential oil from sonicated celery seeds possessed moderate activity against all tested microorganisms (Table 4). The study demonstrated that the oil had a high antimicrobial effect against food-borne pathogens S. aureus. The limonene, pinene (-α, -β), and selinene (-α, -β) are responsible for the biological activity of celery seeds oil [6,18]. The essential oils with a high limonene content like an orange essential oil (Citrus aurantium dulcis), a Tahiti lime essential oil (Citrus limonum), or a caraway essential oil (Carum carvi) inhibited the growth of S. aureus with MIC90% values of 16.5, 14.9 mg/mL, and 1.0 μL/mL, respectively [19,20]. Due to virulence and high antimicrobial resistance, S. aureus is a common cause of infections and is a serious problem in clinical medicine. Other tested bacteria strains exhibited moderate sensitiveness with MIC values between 10 and 30 μL/mL. Yeast C. albicans were more sensitive than moulds and its growth had been inhibited in a concentration of 30 μL/mL. According to Thakre et al. (2018), limonene has destructive effects on the yeast cell surface, thereby resulting in the induction of apoptosis and strongly inhibits C. albicans growth [21]. Furthermore, Ünal et al. (2012) indicated that limonene (10 µL) exhibited a higher antifungal activity than antibiotic Fungizone (50 µL) against 12 strains of tested yeast [22]. Celery seed essential oil has been found to exhibit a strong inhibitory effect against E. coli and good activity against P. aeruginosa, B. subtilis, and S. aureus [13,23,24]. There is no marked difference in the biological activity of ultrasound pre-treatment celery seed essential oil when compared with essential oil obtained by classical hydrodistillation [13].
Table 4. Antimicrobial activity of essential oil from sonicated celery seeds.
2.7. Antioxidant Activity
The essential oil from sonicated celery seeds was characterized by very weak antioxidant activity. Essential oil at the concentrations between 2.5 and 100 g/L expressed an antioxidant effect and quenched the stable free radical DPPH in a range between 34 and 52% (Table 5). The essential oil showed DPPH radical scavenging activity very similar to the control sample [16]. The concentration of essential oil causing 50% inhibition of DPPH radical equalled 81.63 g/L. By way of comparison, the IC50 value for vitamin C was 0.0044 g/L, for oregano (Oregano vulgare L.) essential oil 0.332–0.501 g/L, and for Chinese fennel (Foeniculum vulgare Mill.) essential oil 15.66 mg/g [25,26,27]. In the literature, celery seed essential oil antioxidant activities were defined as weak or moderate [14,28].
Table 5. Antioxidant activity of essential oil from sonicated celery seeds.
2.8. Aroma Profile
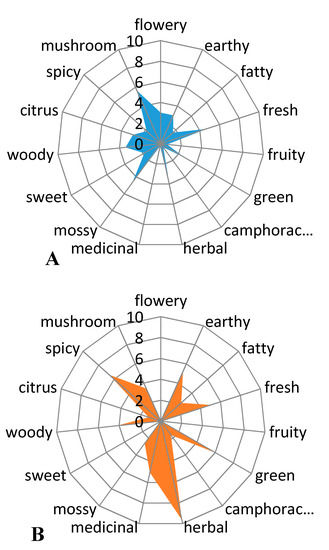
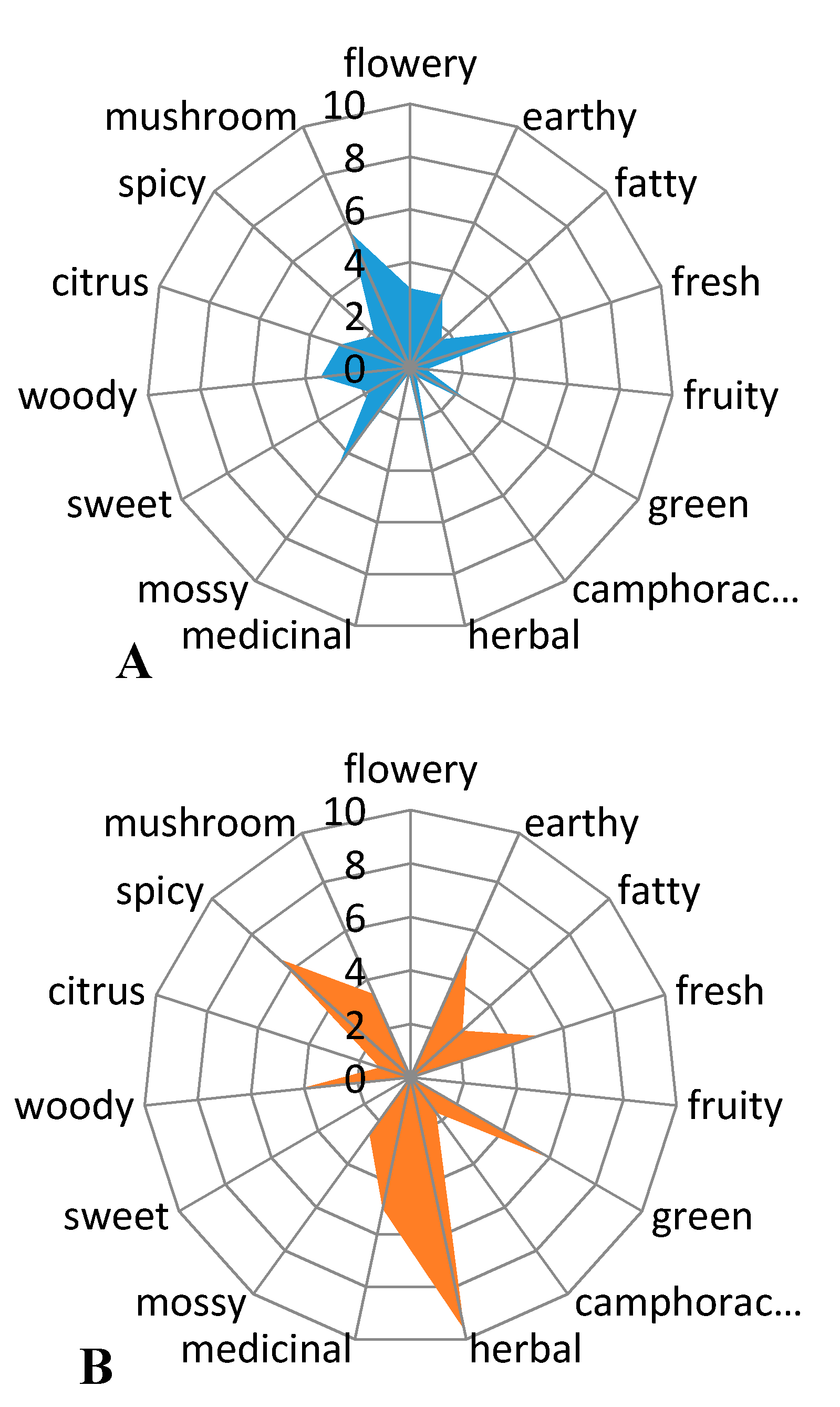
The volatile constituents having a phthalide skeleton: sedanenolide, 3-butylphthalide, sedanolide, and Z-ligustilide are responsible for the celery-like aroma of the essential oil and the whole plant [6,9]. The first two of these were identified in the essential oil from sonicated celery seeds. The odor of essential oil was a celery-like, citrus-like, and vegetable-like with noticeable fresh, green notes, and mirepoix scent. Initial studies of the aroma profile indicated that the sensory impressions could be gender-specific (Figure 3), but further analysis of the topic requires more extensive investigation with more panellists.
Figure 3. Aroma profile of essential oil from sonicated celery seeds assessed by men (A) and by women (B) (n = 3).
Women have sensed more scent notes and they have rated the fragrance as more intense than men. According to women, the smell of celery seed oil was intense herb-like, spicy, and green with fresh, earthy, and medicinal notes. Men had a different opinion from women. From their point of view, the celery oil had fresh, mossy, and mushroom scent. It should be noted that women evaluated that the fragrance of oil was herbal and awarded it the maximum number of points, unlike men, who did not award any points there.
It was found that essential oil from sonicated celery seeds possessed slightly intensive fragrance versus the essential oil obtained a classical hydrodistillation [13]. According to panellists, a citrus-like note is slightly more noticeable in essential oil from sonicated seeds, which may be due to a decrease in the content of sedanenolide and an increase in the content of limonene in the chemical composition.
3. Materials and Methods
3.1. Plant Material
The experimental part of this research was performed with the use of the dried celery seeds (Apium graveolens L.) that showed no germination power and were industrial waste with no utility and economic value. Raw material was donated by the manufacturer as part of a cooperation with the Polish seeds company Przedsiębiorstwo Hodowlano-Nasienne W. Legutko Sp. z o.o (Jutrosin). A voucher specimen is stocked in the manufacturer’s premises (Jutrosin, Poland).
The seeds were grounded in laboratory grinders (Basic A11D, IKA, Staufen, Germany) for 20 s.
3.2. Taguchi Experimental Design Approach
The experiment has been planned according to a DOE approach with the use of the Taguchi method. The selected control factors and their applicable working levels were: time of sonication 5, 20, 50 min; pulse range 0.1, 0.5, 1.0; power control 20, 60,100 %, as well as the content of water 350, 700, 950 mL. The content of water shall mean the water in the sample treated with ultrasounds. The pulse range was: setting 1 meaning continuously switched on, setting 0.6 meaning power discharge 0.6 s, and pause 0.4 s. In this research, the L9 orthogonal array was used, which has nine rows corresponding to the number of tests, with four columns at three levels. Each level of each parameter has been tested three times, which means that the number of required experiments for this module was 27. In the case of the DOE approach, randomness is desired and should be maintained when possible. In relation to this rule, all the trials and repetitions were unbiased and performed in a completely randomized order. Next, the ANOVA was performed (using the Statistica 13.1 software). The Taguchi’s loss function the larger the better was adopted as the best possible loss function for maximizing product yield. In the case of this function, the best quality standard is infinity, and the higher the actual value (the yield of the essential oil), the better. Based on the analysis of Eta values, the best sets of input parameters have been determined and the optimal parameters of the optimized process have been chosen. Last, run confirmation test with optimum conditions have been done in triple repetition. The essential oil obtained from seeds sonicated in optimal conditions was used for further research as the study sample.
The theoretical yield of essential oil was calculated from the formula for Eta in using loss function: Eta=−10⋅log10[(1n⋅∑(1y2i)] , E t a = − 10 · log 10 [ ( 1 n · ∑ ( 1 y i 2 ) ] , where n is the number of iterations and yi is the value of the output variable (the essential oil yield).
3.3. Application of Ultrasounds
The sonication of 100 g of grounded celery seeds (Seeds Company W. Legutko, Poland) was conducted in sonicator UP400S (400W, 24kHz, Hielschier, Germany) with the sound protection box SB1-16 (Hielschier Ultrasonics, Teltow, Germany), electronic timer for controlling the acoustic irradiation duration (Hielschier, Germany), and the titanium sonotrode Tip H3 type (Hielschier Ultrasonics, Teltow, Germany) for transmitting the ultrasound into the liquid. The application of ultrasound was performed according to the L9 orthogonal array containing applicable working levels for each control parameter according to Table 6. The tests were carried out with care to make sure that the sonotrode was always drowned in the same depth.
Table 6. The experimental layout according to the L9 orthogonal array.
3.4. Hydrodistillation Process
The extraction of essential oils was performed by the hydrodistillation process with the use of a modified Deryng apparatus [29]. The 1000 mL of distilled water per 100 g of celery seeds was used. The control sample constituted the essential oil from untreated celery seeds (without ultrasound processes). The yield of essential oil was calculated as an average of three hydrodistillation processes. The percentage increase of yield of essential oil was calculated according to the formula: YUAH−YHDYHD⋅100% Y U A H − Y H D Y H D · 100 % , where: YUAH means the yield of essential oil obtained by ultrasound-assisted hydrodistillation, and YHD means the yield of essential oil obtained by hydrodistillation.
3.5. Physicochemical Parameters
Physicochemical characteristics have been described with an optical rotation α (a polarimeter Autopol IV, Rudolph Research Analytical, Hackettstown, NJ, USA), a refractive index nD20 (an automatic refractometer JI57 Donserv, Rudolph Research Analytical, Hackettstown, NJ, USA), and a density (an automatic densitometer DDM2910, Rudolph Research Analytical, Hackettstown, NJ, USA). Each measurement was repeated three times.
3.6. Gas Chromatography-Mass Spectrometry with Flame Ionization Detection (GC-FID)
Gas chromatography-mass spectrometry analyses were carried out using a Trace GC Ultra gas chromatograph and a DSQ II mass spectrometer (Thermo Electron Corporation, Beverly, MA, USA) with an Rtx-1 column (length 60 m, internal diameter 0.25 mm, film thickness 0.25 mm, Restek Corporation, Bellefonte, PA, USA). A flow divider (an MS-Column Flow Splitter, SGE Analytical Science, Melrose Park, Australia) collected of signals concurrently from two detectors (FID, MSD, Thermo Fisher Scientific, Waltham, MA, USA). The split ratio was 1:20. The temperature program was from 50 °C (3 min) to 300 °C (30 min), at a gradient of 48 °C (21 min). The temperature of the injector (SSL) and detector (FID) was 280 °C and 300 °C, respectively. The helium flowing at a constant pressure of 200 kPa was used as the carrier gas. The mass spectrometer ionization energy was 70 eV, and the ion source temperature was 200 °C. A full scan was conducted in the mass range from 33 to 420. The analysis was repeated three times. The NIST Library, Wiley 8th edition, and the Adams 4th edition were used.
3.7. Similarity Analysis
The essential oil from ultrasound-processed in optimal conditions seeds has been compared in term of chemical composition with the essential oil from celery seeds obtained by hydrodistillation without ultrasound-process. Analysis was performed by the Mann–Whitney test with a significant level of 0.05 and by NIR and MIR spectroscopy.
3.8. Near-Infrared (NIR) and Mid-Infrared (MIR) Spectroscopy
Oil samples were scanned in the infrared spectrometer Nicolet iS50 FT-IR (Thermo Fisher Scientific, Waltham, MA, USA) that allows to generate spectra in two ranges: MIR and NIR. MIR analyses were carried out with a DTGS KBr detector, IR light source, and KBr beamsplitter. The range of scans was from 4000 to 400 cm−1 with a resolution of 4.00 cm−1. The samples were placed in IR Sample Cards (Real Crystal, US) with KBr glass (9.5 mm aperture) and all spectra were accumulated from 32 scans. During NIR measurements there were used: InGaAs detector, white light, and CaF2 beamsplitter. The range of scans was from 12,000–4400 cm−1 with a resolution of 8.00 cm−1. All spectra were accumulated from 32 scans. The samples were placed in the middle of the borosilicate glass tubes (6 × 50 mm) made by Kimble Glass, US. All calculations were made using a commercial analysis software: OMNIC 9.3.30 and TQ Analyst, v. 9.4.45 (Thermo Fisher Scientific, Waltham, MA, USA).
3.9. Antimicrobial Activity Assay
The microbes which were originated from the American Type Culture Collection (ATCC) and the Center of Industrial Microorganisms Collection of the Institute of Fermentation Technology and Microbiology, Lodz University of Technology, Poland, WDCM 105 (LOCK) were examined. Strains tested were both Gram-positive (Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538) and Gram-negative bacteria (Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 15442), as well as fungi (the yeast Candida vini LOCK 0008, the moulds Penicillium expansum LOCK 0535, Aspergillus niger LOCK 16404). Microbial strains were sub-cultured on the medium plate count agar (PCA, Merck, Darmstadt, Germany) for bacteria or potato dextrose agar medium (PDA, BTL, Warsaw, Poland) for fungi, and next, activated through double passaging in Tripticase Soy Broth (TSB, Biocorp, Warsaw, Poland) for bacteria or Sabouraud Dextrose Broth (SDB, BTL, Warsaw, Poland) for fungi. Conditions of incubation were 30 °C (E. coli, B. subtilis), 37 °C (S. aureus, P. aeruginosa) for 24h, and 25 °C for 72 h for fungi. Inoculum of 24-h cultures of each strain was prepared in standard 0.85% sodium chloride solution and adjusted to the final concentration of approximately 107 CFU/mL.
Antimicrobial activity assay was carried out using an impedimetric method (Bactometer M64, bioMerieux, Craponne, France). The celery seed essential oil was diluted in ethyl alcohol (pure P.A., Avantor Performance Materials Polan, Gliwice, Poland) in a ratio of 1:1. Each sample included 0.1 mL of the standardized microorganism inoculum and the essential oil at the examined concentration within the range from 50 to 500 μg/mL. Next, each Bactometer well was filled until a final volume of 1 mL was obtained with the use of the growth medium: general purpose medium (GPM, bioMerieux, Craponne, France) for B. subtilis, S. aureus, and P. aeruginosa, coliform medium (CM, bioMerieux, Craponne, France) for E. coli, and yeast and mould medium (YMM, bioMerieux, Craponne, France) for A. niger, P. expansum, and C. vini. In the negative controls, instead of the essential oil, 0.5 μg/mL of novobiocin for bacteria or 0.2 μg/mL of cycloheximide for yeast and moulds were added. Positive controls did not contain essential oil, but only 0.1 mL of cell standardized suspension in 0.9 mL of the appropriate medium. The microorganisms were incubated for 72 h at their optimal growth temperatures (30 °C for E. coli, B. subtilis, 37 °C for S. aureus, P. aeruginosa, and 25 °C for fungi). Next, the tested strains were checked for their viability by streaking on the PCA medium. Plates were incubated for 72 h for bacteria and 120 h for fungi at the optimal growth temperatures. The results which were the mean value of three measurements were presented as MIC and MBC.
3.10. Antioxidant Activity
Radical scavenging activity was examined using the DPPH assay. First, the 1-μM DPPH (Sigma-Aldrich, Hamburg, Germany) solution in methanol (pure p.a., Chempur, Poland) was prepared. Next, the 200 μL of DPPH was added to 100 μL of the solution of essential oil in methanol at concentrations of 2.5, 5, 10, 20, 50, and 100 g/L, respectively. The analysis was performed using a 96-well polystyrene plate (Nest Biotechnology, Wuxi, China). The samples were incubated in the dark at room temperature for 30 min. As the reference, a methanol solution of Trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, Sigma-Aldrich, Saint Louis, MO, USA) was used. The absorbance of the DPPH radical was spectrophotometrically analyzed at a wavelength of 517 nm (Modular Multimode Microplate Reader TriStar2 S, Berthold Technologies, Oak Ridge, TN, USA). The results were shown as the percentage of inhibition of the DPPH radicals calculated according to the formula: = (A0−A1)A0⋅100 = ( A 0 − A 1 ) A 0 · 100 , where, A0 is the absorbance of the control sample (DPPH), and A1 is the absorbance of the sample with the essential oil. The results were expressed as the arithmetic mean value of the three consecutive measurements with a standard deviation value.
3.11. Aroma Profile
The aroma profile of essential oil was performed by untrained analytical teams of five males and five females. A hedonic ten-point scale test was applied. After inhalation, the panellists marked the intensity scale of aroma from 1 to 10 points, where 0 is none or not perceptible intensiveness, and 10 is strong intensiveness. The distinguishing features as fatty, mossy, green, camphoraceous, herbal, citrus, fruity, earthy, flowery, fresh, medicinal, mushroom, sweet, spicy, and woody were evaluated. Sensory hallmarks were according to El-Zaeddi et al. (2016) with modifications [30]. The results were shown as the average of all measurements.
4. Conclusion
The presented research provides important information about the non-conventional method for obtaining essential oils, which is ultrasound-assisted hydrodistillation. This technique provides a high yield of obtained essential oil and with respect for pro-environmental aspects. The product obtained has a chemical composition very similar to essential oil extracted by hydrodistillation. The pre-treatment of the seeds by ultrasounds did not result in a loss in their quality. The essential oil from sonicated celery seeds could be a replacement for ‘traditional’ celery seed essential oil and be used in the cosmetics and food industry as a flavoring agent and adjuvant. In the future, it could substitute for synthetic pesticides and food preservatives.
Author Contributions
Conceptualization, A.K.-S. and K.Ś.; Formal analysis, J.Z.; Investigation, J.Z. and R.G.; Methodology, J.Z., A.K.-S., and K.Ś.; Resources, A.K.-S. and K.Ś.; Software, J.Z. and R.G.; Supervision, A.K.-S. and K.Ś.; Visualization, J.Z.; Writing—original draft, J.Z.; Writing—review & editing, J.Z., A.K.-S., R.G., and K.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research had no funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Majewska, M.; Kunicka-Styczyńska, A.; Gruska, R. The effect of ultrasound-assisted maceration on the bioactivity, chemical composition and yield of essential oil from waste carrot seeds (Daucus carota). J. Essent. Oil Bear. Plants 2014, 17, 1075–1086. [Google Scholar] [CrossRef]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques for extraction of essential oils from plants: A review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Zor, Ş.D.; Bat, M.; Peksel, A.; Alpdoğan, G. Optimization of ultrasound-assisted extraction of antioxidants from Apium graveolens L. seeds using response surface methodology. J. Turk. Chem. Soc. Section A Chem. 2017, 4, 915–930. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A review of the antioxidant activity of celery (Apium graveolens L.). J. Evid. Based Complementary Altern. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salehi, B.; Venditti, A.; Frezza, C.; Yücetepe, A.; Altuntaȿ, Ü.; Uluata, S.; Butnariu, M.; Sarac, I.; Shaheen, S.; Petropoulos, S.A.; et al. Apium plants: Beyond simple food and phytopharmacological applications. Appl. Sci. 2019, 9, 3547. [Google Scholar] [CrossRef][Green Version]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef][Green Version]
- Miljanović, A.; Bielen, A.; Grbin, D.; Marijanović, Z.; Andlar, M.; Rezić, T.; Roca, S.; Jerković, I.; Vikić-Topić, D.; Dent, M. Effect of enzymatic, ultrasound, and reflux extraction pretreatments on the chemical composition of essential oils. Molecules 2020, 25, 4818. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Chen, C.-T.; Wang, J.-J.; Kuo, Y.-H.; Li, C.-C.; Hsieh, L.-C.; Wu, C.-C. Sedanolide induces autophagy through the PI3K, p53 and NF-κB signaling pathways in human liver cancer cells. Int. J. Oncol. 2015, 47, 2240–2246. [Google Scholar] [CrossRef][Green Version]
- Kowalski, R.; Wawrzykowski, J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour Fragr. J. 2009, 24, 69–74. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Jamroz, J.; Nawrocka, A.; Metyk, D. Effect of the ultrasound-assisted preliminary maceration on the efficiency of the essential oil distillation from selected herbal raw materials. Ultrason. Sonochem. 2015, 24, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.; Gagoś, M.; Kowalska, G.; Pankiewicz, U.; Sujka, M.; Mazurek, A.; Nawrocka, A. Effects of ultrasound technique on the composition of different essential oils. J. Anal. Methods Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, J.A.; Kunicka-Styczyńska, A.; Śmigielski, K. Biological, chemical, and aroma profiles of essential oil from waste celery seeds (Apium graveolens L.). J. Essent. Oil Res. 2020. [Google Scholar] [CrossRef]
- Kiralan, M.; Bayrak, A.; Abdulaziz, O.F.; Özbucak, T. Essential oil composition and antiradical activity of the oil of Iraq plants. Nat. Prod. Res. 2012, 26, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A.; Hussein, M.S. Effect of cattle and liquid manures on essential oil and antioxidant activities of celery (Apium graveolens L.) fruits. J. Essent. Oil Bear. Plants 2012, 15, 97–107. [Google Scholar] [CrossRef]
- Hassanen, N.H.; Eissa, A.M.F.; Hafez, S.A.M.; Mosa, E.A.M. Antioxidant and antimicrobial activity of celery (Apium graveolens) and coriander (Coriandrum sativum) herb and seed essential oils. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 284–296. [Google Scholar]
- Zhang, X.; Xiao, H.; Xu, Q.; Li, X.; Wang, J.; Liang, X. Characterization of phthalides in Ligusticum chuanxiong by liquid chromatographic-atmospheric pressure chemical ionization-mass spectrometry. J. Chromatogr. Sci. 2003, 41, 428–433. [Google Scholar] [CrossRef][Green Version]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.H.S. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev. Res. 2015, 8, 86–95. [Google Scholar] [CrossRef][Green Version]
- Andrade, B.F.M.T.; Barbosa, L.N.; Probst, I.S.; Fernandes, A.J. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Tarek, N.; Hassan, H.M.; Abdel-Ghani, S.M.M.; Radwan, I.A.; Hammouda, O.; El-Gendy, A.O. Comparative chemical and antimicrobial study of nine essential oils obtained from medicinal plants growing in Egypt. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 149–156. [Google Scholar] [CrossRef][Green Version]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. 2018, 56, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Ünal, M.U.; Uçan, F.; Sener, A.; Dinçer, S. Research on antifungal and inhibitory effects of dl-limonene on some yeasts. Turk. J. Agric. For. 2012, 36, 576–582. [Google Scholar] [CrossRef]
- Baananou, S.; Bouftira, I.; Mahmoud, A.; Boukel, K.; Marongiu, B.; Boughattas, N.A. Antiulcerogenic and antibacterial activities of Apium graveolens essential oil and extract. Nat. Prod. Res. 2013, 27, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Din, Z.U.; Shad, A.A.; Bakht, J.; Ullah, I.; Jan, S. In vitro antimicrobial, antioxidant activity and phytochemical screening of Apium graveolens. Pak. J. Pharm. Sci. 2015, 28, 1699–1704. [Google Scholar]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef][Green Version]
- Ahmed, A.F.; Shi, M.; Liu, C.; Kang, W. Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci. Hum. Wellness 2019, 8, 67–72. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Śmigielski, K.; Raj, A.; Krosowiak, K.; Gruska, R. Chemical composition of the essential oil of Lavandula angustifolia cultivated in Poland. J. Essent. Oil Bear. Plants 2009, 12, 338–347. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean regions of Spain. Foods 2016, 5. [Google Scholar] [CrossRef][Green Version]
Figure 1. The graphs of average Eta versus input levels of optimized parameters. Solid line—value of Eta, dashed line—±2∙standard error, 1,2,3—input levels for each parameters (content of water: 1–350 mL, 2–700 mL, 3–950 mL; power control amplitude 1–20%, 2–60%, 3–100%); pulse range: 1–0.1, 2–0.5, 3–1; sonication time: 1–5 min, 2–20 min, 3–50 min); n = 3.
Figure 2. GC-MS chromatogram of the essential oil from sonicated celery seeds (Apium graveolens L.). For peak identification, see Table 3.
Figure 3. Aroma profile of essential oil from sonicated celery seeds assessed by men (A) and by women (B) (n = 3).
Table 1. Statistical analysis of input factors for ultrasonic pre-treatment of celery seeds according to the Taguchi method.
| Test No. | Sonication Time (min) | Pulse Range | Power Control Amplitude (%) | Content of Water (mL) | Essential Oil Yield (g/100 g of Seeds) | Eta |
|---|---|---|---|---|---|---|
| 1 | 5 | 0.1 | 20 | 350 | 1.456 ± 0.011 | 3.318555 |
| 2 | 5 | 0.5 | 60 | 700 | 1.978 ± 0.048 | 5.922812 |
| 3 | 5 | 1 | 100 | 950 | 1.731 ± 0.035 | 4.763080 |
| 4 | 20 | 0.1 | 60 | 950 | 1.949 ± 0.029 | 5.797067 |
| 5 | 20 | 0.5 | 100 | 350 | 1.690 ± 0.011 | 4.557621 |
| 6 | 20 | 1 | 20 | 700 | 1.884 ± 0.015 | 5.501434 |
| 7 | 50 | 0.1 | 100 | 700 | 1.908 ± 0.061 | 5.607108 |
| 8 | 50 | 0.5 | 20 | 950 | 1.961 ± 0.045 | 5.846586 |
| 9 | 50 | 1 | 60 | 350 | 1.927 ± 0.043 | 5.697718 |
Table 2. Expected S/N ratio (Eta) under optimum conditions.
| Factor | Level | Effect Size | Standard Error |
|---|---|---|---|
| Contents of water [mL] | 2 | 0.453565 | 0.097524 |
| Power control amplitude [%] | 2 | 0.582312 | 0.097524 |
| Pulse range | 2 | 0.218786 | 0.097524 |
| Sonication time [min] | 3 | 0.493584 | 0.097524 |
| Expected S/N ratio | 6.971801 | ||
Table 3. Composition of essential oil from ultrasounds pre-treated celery seeds (GC-MS).
| No. | Chemical Compound | RT (min) | RIE | RIL | Area (%) | |
|---|---|---|---|---|---|---|
| EOUAH | EOHD * | |||||
| 1 | α-pinene | 12.8 | 937 | 940 | 0.1 | 0 |
| 2 | sabinene | 14.4 | 967 | 965 | tr | 0 |
| 3 | β-pinene | 15.0 | 980 | 972 | 0.8 | 0 |
| 4 | β-myrcene | 15.5 | 993 | 992 | 0.6 | 0.1 |
| 5 | p-cymene | 16.3 | 1013 | 1011 | tr | 0 |
| 6 | limonene | 16.8 | 1026 | 1027 | 76.9 | 1 |
| 7 | β-linalool | 19.2 | 1085 | 1082 | tr | 0.1 |
| 8 | octen-1-ol acetate | 19.5 | 1100 | 1102 | tr | 0 |
| 9 | trans-p–mentha-2,8-dien-1-ol | 19.9 | 1103 | 1103 | 0.1 | 0 |
| 10 | limona ketone | 20.0 | 1106 | 1105 | tr | 0 |
| 11 | cis-p-mentha-2,8-dien-1-ol | 20.4 | 1116 | 1116 | 0.2 | 0.1 |
| 12 | limonene oxide | 21.3 | 1138 | 1138 | 0.1 | 0 |
| 13 | pentylbenzene | 21.5 | 1144 | 1146 | 0.7 | −0.1 |
| 14 | 1-pentylcyclohexa-1,3-diene | 21.7 | 1148 | 1156 | 0.1 | 0 |
| 15 | p-mentha-1,8-dien-4-ol | 22.1 | 1167 | 1174 | tr | 0 |
| 16 | trans-isocarveol | 22.5 | 1169 | 1175 | tr | 0 |
| 17 | cis-dihydrocarvone | 22.6 | 1171 | 1167 | 0.1 | 0 |
| 18 | α-terpineol | 22.9 | 1179 | 1179 | 0.1 | 0 |
| 19 | dihydrocarveol | 23.0 | 1181 | 1181 | 0.1 | 0 |
| 20 | α-curcumene | 23.9 | 1479 | 1472 | tr | 0 |
| 21 | β-selinene (β-eudesmene) | 34.0 | 1487 | 1491 | 9.7 | 0.4 |
| 22 | α-selinene (α-eudesmene) | 34.2 | 1494 | 1500 | 1.4 | 0.1 |
| 23 | 7-epi-α-selinene | 35.4 | 1532 | 1526 | tr | 0 |
| 24 | selina-3,7(11)-diene | 35.5 | 1540 | 1535 | tr | 0 |
| 25 | hedycariol | 36.1 | 1545 | 1541 | tr | 0 |
| 26 | β-caryophyllene oxide | 36.7 | 1573 | 1576 | 0.5 | 0 |
| 27 | humulene epoxide 2 | 37.5 | 1597 | 1601 | tr | 0 |
| 28 | 3-butylphthalide | 38.0 | 1626 | 1629 | 3.6 | 0.5 |
| 29 | β-eudesmol | 38.7 | 1639 | 1644 | 0.5 | 0.1 |
| 30 | sedanenolide (senkyunolide A) | 39.9 | 1698 | 1701 | 3.4 ** | −1.1 |
| total | 99.0 | |||||
| monoterpenes | 78.4 | |||||
| monoterpenoids | 0.7 | |||||
| sesquiterpenes | 11.1 | |||||
| sesquiterpenoids | 1.0 | |||||
| other hydrocarbons | 7.8 | |||||
tr—trace, EOUAH—the essential oil obtained by ultrasound-assisted hydrodistillation, EOHD—the essential oil obtained by hydrodistillation, RT—retention time, RIE and RIL—experimental and literature (the NIST Standard Reference Database Number 69) retention index, *—the difference in the content expressed in percentage point in relation to Dąbrowska et al. (2020) [13], where 0 means no differences in chemical composition, **—a chemical compound that content has changed statistically significantly.
Table 4. Antimicrobial activity of essential oil from sonicated celery seeds.
| Strain | MIC (μL/mL) | MBC/MFC * (μL/mL) |
|---|---|---|
| Escherichia coli ATCC 1627 | 10 | 70 |
| Psudomonas aeruginosa ATCC 1555 | 30 | 100 |
| Bacillus subtilis ATCC 6633 | 20 | 150 |
| Staphylococcus aureus ATCC 1803 | 3 | 20 |
| Aspergillus niger LOCK 16404 | 40 | >300 * |
| Penicillum expansum LOCK 0535 | 40 | >300 * |
| Candida vini LOCK 0008 | 30 | 120 * |
MIC—minimal inhibitory concentration, MBC—minimal bactericidal concentration, MFC—minimal fungicidal concentration. SD (the standard deviations) equalled 0, (n = 3).
Table 5. Antioxidant activity of essential oil from sonicated celery seeds.
| Concentration of Essential Oil (g/L) | 2.5 | 5.0 | 10.0 | 20.0 | 50.0 | 100.0 |
|---|---|---|---|---|---|---|
| DPPH radicals scavenging effect (%) | 34.5 ± 0.3 | 35.3 ± 0.3 | 37.9 ± 0.3 | 39.6 ± 0.3 | 44.8 ± 0.4 | 52.9 ± 0.4 |
| Trolox Equivalents (µg/mL) | 5.6 ± 0.3 | 5.8 ± 0.3 | 6.4 ± 0.3 | 6.9 ± 0.3 | 8.2 ± 0.4 | 10.2 ± 0.4 |
| IC50 parameter (g/L) | 81.6 | |||||
Table 6. The experimental layout according to the L9 orthogonal array.
| Test No. | Sonication Time (min) | Pulse Range | Power Control Amplitude (%) | Content of Water (mL) |
|---|---|---|---|---|
| 1 | 5 | 0.1 | 20 | 350 |
| 2 | 5 | 0.5 | 60 | 700 |
| 3 | 5 | 1 | 100 | 950 |
| 4 | 20 | 0.1 | 60 | 950 |
| 5 | 20 | 0.5 | 100 | 350 |
| 6 | 20 | 1 | 20 | 700 |
| 7 | 50 | 0.1 | 100 | 700 |
| 8 | 50 | 0.5 | 20 | 950 |
| 9 | 50 | 1 | 60 | 350 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MDPI and ACS Style
Zorga, J.; Kunicka-Styczyńska, A.; Gruska, R.; Śmigielski, K. Ultrasound-Assisted Hydrodistillation of Essential Oil from Celery Seeds (Apium graveolens L.) and Its Biological and Aroma Profiles. Molecules 2020, 25, 5322. https://doi.org/10.3390/molecules25225322
AMA Style
Zorga J, Kunicka-Styczyńska A, Gruska R, Śmigielski K. Ultrasound-Assisted Hydrodistillation of Essential Oil from Celery Seeds (Apium graveolens L.) and Its Biological and Aroma Profiles. Molecules. 2020; 25(22):5322. https://doi.org/10.3390/molecules25225322
Chicago/Turabian Style
Zorga, Justyna, Alina Kunicka-Styczyńska, Radosław Gruska, and Krzysztof Śmigielski. 2020. “Ultrasound-Assisted Hydrodistillation of Essential Oil from Celery Seeds (Apium graveolens L.) and Its Biological and Aroma Profiles” Molecules 25, no. 22: 5322. https://doi.org/10.3390/molecules25225322
Find Other Styles
Article Metrics
Yes
Citations
Crossref
11
Web of Science
9
Scopus
10
PubMed
2
PMC
2
Google Scholar
[click to view]
No
Article Access Statistics
Created with Highcharts 4.0.4 Article access statistics Article Views 12. Dec 13. Dec 14. Dec 15. Dec 16. Dec 17. Dec 18. Dec 19. Dec 20. Dec 21. Dec 22. Dec 23. Dec 24. Dec 25. Dec 26. Dec 27. Dec 28. Dec 29. Dec 30. Dec 31. Dec 1. Jan 2. Jan 3. Jan 4. Jan 5. Jan 6. Jan 7. Jan 8. Jan 9. Jan 10. Jan 11. Jan 12. Jan 13. Jan 14. Jan 15. Jan 16. Jan 17. Jan 18. Jan 19. Jan 20. Jan 21. Jan 22. Jan 23. Jan 24. Jan 25. Jan 26. Jan 27. Jan 28. Jan 29. Jan 30. Jan 31. Jan 1. Feb 2. Feb 3. Feb 4. Feb 5. Feb 6. Feb 7. Feb 8. Feb 9. Feb 10. Feb 11. Feb 12. Feb 13. Feb 14. Feb 15. Feb 16. Feb 17. Feb 18. Feb 19. Feb 20. Feb 21. Feb 22. Feb 23. Feb 24. Feb 25. Feb 26. Feb 27. Feb 28. Feb 1. Mar 2. Mar 3. Mar 4. Mar 5. Mar 6. Mar 7. Mar 8. Mar 9. Mar 10. Mar 11. Mar 0 500 1000 1500 2000 2500
For more information on the journal statistics, click here.
Multiple requests from the same IP address are counted as one view.
Home /
Celery Essential Oil – CO2 Extracted (Apium Graveolens)
SKU: CELERY-5ml
Regular price$10.97
/
Shipping calculated at checkout.
- Botanical Name: Apium Graveolens
- Plant Part: Herb
- Method of Extraction: CO2 Extracted
- Country of Origin: India
- Color/Consistency: Brown/Thin Consistency
- Aroma: Intense, Warm, Herbaceous
- Perfumery Note: Middle
- Main Chemical Components: Sedanolide (49.47%), Limonene (24.03), beta-Selinene (7.45%)
Description
Celery Essential Oil is obtained by supercritical CO2 extraction from the celery plant. Celery is a widespread vegetable noted in the aromatherapy industry for many health benefits, including lowering blood pressure, promoting relaxation and sound sleep, and increasing strength and energy.
Celery Essential Oil is popular in perfumery due to its unique aromatic note. It also has a number of therapeutic properties inherited from the celery plant, such as its support for digestive health, its calming and soothing effects, and its ability to lessen the perception of pain.
Properties: Antispasmodic, Carminative, Nervine, Sedative, Tonic Perfumery Note: Middle Aroma: Intense, Warm, Herbaceous Blends with: Basil, Turmeric, Black Pepper, Ginger, Lavender, Tea Tree, Pine, Roman Chamomile, Orange
Benefits
Digestive health… Celery Essential Oil is commonly used to help with numerous digestive issues, such as indigestion, flatulence, and balancing stomach acids. It has also been connected to natural weight loss, as it appears to reduce cravings for sugary foods.
Lower back pain… Celery Essential Oil has anti-inflammatory properties and known to be calming to the nerves. As a consequence, preparations using this essential oil can reduce lower back pain and has been proposed as a treatment of sciatica.
Anti-anxiety… Because of its calming effect on the nervous system, Celery Essential Oil is used to help reduce mental fatigue, stress, and anxiety. Celery Essential Oil has also been used to resolve insomnia and promote deep, restorative sleep.
Directions
How To Use Essential Oils
Recipes
Lower back massage balm
1 oz. Argan Oil
4 drops Roman Chamomile Essential Oil
4 drop Celery Essential Oil
4 drop Turmeric Essential Oil
Blend the oils together and massage onto the lower back.
Curing digestive upsets
1 oz. Argan Oil
4 drops Ginger Essential Oil
2 drops Celery Essential Oil
4 drops Peppermint Essential Oil
Mix the oils together and massage the blend onto your belly.
Calming and uplifting diffusion
Grapefruit Essential Oil
Celery Essential Oil
Lavender Essential Oil
Follow the instructions for your diffuser about how many drops of essential oil to use.
Test Report
CLICK TO VIEW GCMS
Articles
Healthy Digestion with Essential Oils
Size
5ml 10ml 30ml
Pricing at a Glance:
- 5ml – $10.97
- 10ml – $12.97
- 30ml – $29.97
- In stock, ready to ship
- Inventory on the way
Quantity
− +
Add to cart
Buy now with ShopPayBuy with
More payment options
Add to Wishlist
5ml – $10.97 USD 10ml – $12.97 USD 30ml – $29.97 USD [{“id”:40286038622261,”title”:”5ml”,”option1″:”5ml”,”option2″:null,”option3″:null,”sku”:”CELERY-5ml”,”requires_shipping”:true,”taxable”:true,”featured_image”:{“id”:30650739851317,”product_id”:6743050649653,”position”:2,”created_at”:”2021-12-10T16:53:16-10:00″,”updated_at”:”2022-02-16T12:35:25-10:00″,”alt”:”Celery Essential Oil – CO2 Extracted – Miracle Botanicals Essential Oils”,”width”:1080,”height”:1080,”src”:”https:\/\/cdn.shopify.com\/s\/files\/1\/0554\/1868\/3445\/products\/Celery-CO2-Photo-5ml.jpg?v=1645050925″,”variant_ids”:[40286038622261]},”available”:true,”name”:”Celery Essential Oil – CO2 Extracted (Apium Graveolens) – 5ml”,”public_title”:”5ml”,”options”:[“5ml”],”price”:1097,”weight”:0,”compare_at_price”:null,”inventory_management”:null,”barcode”:null,”featured_media”:{“alt”:”Celery Essential Oil – CO2 Extracted – Miracle Botanicals Essential Oils”,”id”:22949048549429,”position”:2,”preview_image”:{“aspect_ratio”:1.0,”height”:1080,”width”:1080,”src”:”https:\/\/cdn.shopify.com\/s\/files\/1\/0554\/1868\/3445\/products\/Celery-CO2-Photo-5ml.jpg?v=1645050925″}},”requires_selling_plan”:false,”selling_plan_allocations”:[]},{“id”:40286038556725,”title”:”10ml”,”option1″:”10ml”,”option2″:null,”option3″:null,”sku”:”CELERY-10ml”,”requires_shipping”:true,”taxable”:true,”featured_image”:{“id”:30650739785781,”product_id”:6743050649653,”position”:1,”created_at”:”2021-12-10T16:53:16-10:00″,”updated_at”:”2022-02-16T12:35:24-10:00″,”alt”:”Celery Essential Oil – CO2 Extracted – Miracle Botanicals Essential Oils”,”width”:1080,”height”:1080,”src”:”https:\/\/cdn.shopify.com\/s\/files\/1\/0554\/1868\/3445\/products\/Celery-CO2-Photo-10ml.jpg?v=1645050924″,”variant_ids”:[40286038556725]},”available”:true,”name”:”Celery Essential Oil – CO2 Extracted (Apium Graveolens) – 10ml”,”public_title”:”10ml”,”options”:[“10ml”],”price”:1297,”weight”:0,”compare_at_price”:null,”inventory_management”:null,”barcode”:null,”featured_media”:{“alt”:”Celery Essential Oil – CO2 Extracted – Miracle Botanicals Essential Oils”,”id”:22949048483893,”position”:1,”preview_image”:{“aspect_ratio”:1.0,”height”:1080,”width”:1080,”src”:”https:\/\/cdn.shopify.com\/s\/files\/1\/0554\/1868\/3445\/products\/Celery-CO2-Photo-10ml.jpg?v=1645050924″}},”requires_selling_plan”:false,”selling_plan_allocations”:[]},{“id”:40286038589493,”title”:”30ml”,”option1″:”30ml”,”option2″:null,”option3″:null,”sku”:”CELERY-30ml”,”requires_shipping”:true,”taxable”:true,”featured_image”:{“id”:30650739818549,”product_id”:6743050649653,”position”:3,”created_at”:”2021-12-10T16:53:16-10:00″,”updated_at”:”2022-02-16T12:35:28-10:00″,”alt”:”Celery Essential Oil – CO2 Extracted – Miracle Botanicals Essential Oils”,”width”:1080,”height”:1080,”src”:”https:\/\/cdn.shopify.com\/s\/files\/1\/0554\/1868\/3445\/products\/Celery-CO2-Photo-30ml.jpg?v=1645050928″,”variant_ids”:[40286038589493]},”available”:true,”name”:”Celery Essential Oil – CO2 Extracted (Apium Graveolens) – 30ml”,”public_title”:”30ml”,”options”:[“30ml”],”price”:2997,”weight”:0,”compare_at_price”:null,”inventory_management”:null,”barcode”:null,”featured_media”:{“alt”:”Celery Essential Oil – CO2 Extracted – Miracle Botanicals Essential Oils”,”id”:22949048516661,”position”:3,”preview_image”:{“aspect_ratio”:1.0,”height”:1080,”width”:1080,”src”:”https:\/\/cdn.shopify.com\/s\/files\/1\/0554\/1868\/3445\/products\/Celery-CO2-Photo-30ml.jpg?v=1645050928″}},”requires_selling_plan”:false,”selling_plan_allocations”:[]}]
Pickup available at Miracle Botanicals
Usually ready in 24 hours
View store information
Celery Essential Oil – CO2 Extracted (Apium Graveolens)
5ml
Close cart
Miracle Botanicals
Pickup available, usually ready in 24 hours
15-1929 32nd Avenue Keeau HI 96749 United States
+18886657719
Description
- Botanical Name: Apium Graveolens
- Plant Part: Herb
- Method of Extraction: CO2 Extracted
- Country of Origin: India
- Color/Consistency: Brown/Thin Consistency
- Aroma: Intense, Warm, Herbaceous
- Perfumery Note: Middle
- Main Chemical Components: Sedanolide (49.47%), Limonene (24.03), beta-Selinene (7.45%)
Chia sẻ Chia sẻ trên Facebook Tweet Tweet trên Twitter Ghim nó Ghim trên Pinterest
- Miễn phí vận chuyển đến mọi nơi ở Hoa Kỳ
Video Celery hydrosol (Apium graveolens) [mới nhất 2023], 6 lưu ý nên biết
Lưu ý: Nội dung trong bài viết này chỉ mang tính chất tham khảo. Thông tin được tổng hợp tự động bởi AI. Đề nghị các bạn không làm theo dưới mọi hình thức.